Available online 29 June 2022
Abstract
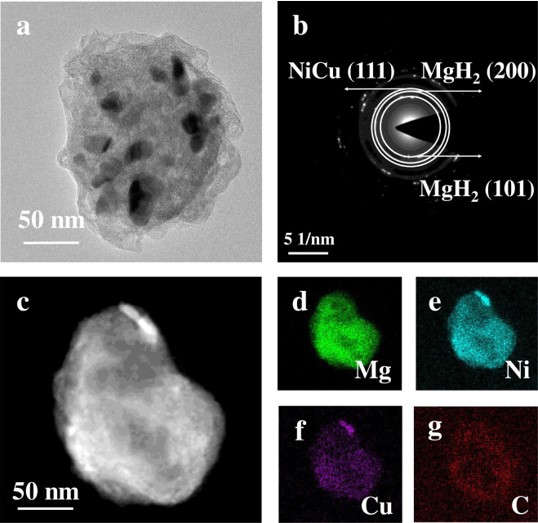
The hydrogen absorption/desorption kinetic properties of MgH2 can be effectively enhanced by doping specific catalysts. In this work, MOFs-derived NiCu@C nanoparticles (∼15 nm) with regular core-shell structure were successfully prepared and introduced into MgH2 (denoted as MgH2NiCu@C). The onset and peak temperatures of hydrogen desorption of MgH2–11 wt.% NiCu@C are 175.0 °C and 282.2 °C, respectively. The apparent activation energy of dehydrogenated reaction is 77.2 ± 4.5 kJ/mol for MgH2–11 wt.% NiCu@C, which is lower than half of that of the as-milled MgH2. Moreover, MgH2–11 wt.% NiCu@C displays great cyclic stability. The strengthening “hydrogen pumping” effect of reversible solid solutions Mg2Ni(Cu)/Mg2Ni(Cu)H4 is proposed to explain the remarkable improvement in hydrogen absorption/desorption kinetic properties of MgH2. This work offers a novel perspective for the design of bimetallic nanoparticles and beyond for application in hydrogen storage and other energy related fields.
Keywords